
Everyone is leaving Plexus becasue the income promises shared by Plexus distributors on social media, like paying off college debt, aren’t true. In reality, less than 1% of distributors make six figures, and more than three-quarters earn less than $500 a year, as reported by TINA.org.
Like everyone else, I wouldn’t want to join a company that doesn’t seem reliable. Plexus has had issues with things like its MLM structure, not delivering on promises, pricey products, pushing you to make money from friends, and tricky marketing. Plexus has made false promises and used tricks that have caused people to lose money in the end.
So, why is everyone leaving Plexus? A big reason is the company’s reputation. The FTC has gotten over 800 complaints about Plexus. A lot of these complaints are from people who say Plexus kept charging them for stuff they didn’t even want. I’ve done some digging online and talked to former Plexus Brand ambassadors, and this article breaks down why people are leaving Plexus. We’ll see whether Plexus is a pyramid scheme, explore why it got banned in certain countries, and understand how Plexus Pink Drink reviews influence why people decide to quit Plexus.
Why Is Everyone Leaving Plexus?
People leave Plexus for a variety of reasons including health concerns, personal circumstances, dissatisfaction with results or the MLM business model, financial constraints, dietary preferences, medical advice, a shift towards natural diet choices, or lack of community support. I reached out to Morgan, one of the former Plexus Ambassadors that I’ve followed on her social media, to understand her Plexus journey, starting with her initial experience. She recalled, “My journey with Plexus began in December 2014. I was initially amazed by the changes; I felt more energetic, slept better, and my overall health seemed to improve significantly. I started with the Triplex and was actively sharing my positive experiences.”
Curious about what led to her first departure from Plexus, I asked Morgan. She explained, “Life just became too overwhelming. After a miscarriage, I was struggling emotionally and physically. Despite the benefits, I couldn’t keep up with Plexus amidst everything else in my life.” I wondered how her health changed after quitting. Morgan shared, “My health deteriorated after I stopped. I suffered from headaches, fatigue, and weight gain, a stark contrast to how I felt while on Plexus.”
On rejoining Plexus during pregnancy, Morgan said, “During a dinner with a friend, I expressed how awful I felt during my pregnancy. She suggested getting back on Plexus. Desperate to feel better and after consulting with my OB and lactation consultant, I restarted, hoping for the same positive effects.” Regarding Plexus’s impact on her pregnancy and postpartum experience, Morgan noted, “Post-rejoining, my energy improved, and my acid reflux became manageable. My baby was born healthy, and my breastfeeding experience was positive, which I attribute to Plexus.”
I asked Morgan why she decided to leave Plexus again. She revealed, “Despite the benefits, I realized Plexus wasn’t a sustainable solution for me. The demands of being an ambassador, combined with the realization that long-term health requires more than just supplements, led me to seek a more balanced approach to health and wellness.”
In our conversation, Morgan revealed that she wasn’t alone in leaving Plexus; many of her former colleagues had also departed, each for their personal reasons. She shared, “A lot of us started to question the real benefits of Plexus. We didn’t see the results we were promised, and it hit us hard, both emotionally and financially. The pressure to keep selling and recruiting was overwhelming, and when you add in the ethical concerns about how the products were marketed, it just didn’t sit right with many of us.”
Lack of Verifiable Benefits
Based on what I’ve read in Plexus reviews, many people stop using Plexus products because they don’t see any real benefits. Despite all the marketing hype and positive testimonials, a lot of people find that their actual results don’t match the promises.
People often hear amazing stories about Plexus, where people have experienced incredible health improvements and lost a lot of weight. These stories create big expectations for newcomers, especially when they hear personal success stories from others. In my opinion, it’s challenging to justify spending your hard-earned cash on Plexus products when there’s a lack of solid evidence backing their supposed benefits. Many people are starting to question whether the investment is worth it, especially when they don’t see the promised results.
Financial Strain
Many people have joined Plexus hoping for financial relief, but the reality can be tough. Initially drawn in by the products, they soon realize how pricey it can be to keep up. For example, the Tri-plex combo and a multivitamin can easily cost around $200 each month.
Plexus talks about potential earnings, but in reality, most people, especially those at lower levels, make very little. Unfortunately, Plexus doesn’t openly share its US Income Disclosure Statement. Despite claims of unlimited earning potential and a 50% payout, the majority of individuals end up with just $30 to $50 per month, as shown in this chart.
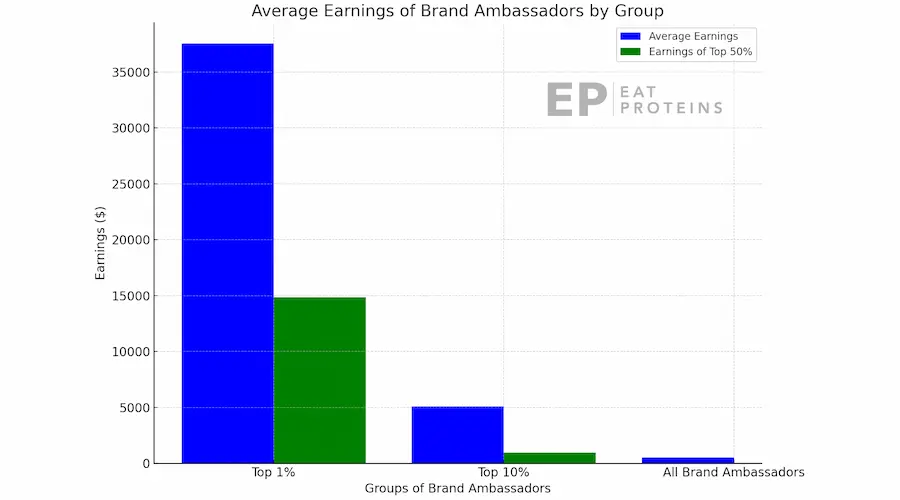
Morgan shared that the monthly expenses for products are just a small part of the overall cost. Ambassadors also have to bear additional expenses like fees for annual conventions, costs for marketing materials, and charges for training sessions. Morgan shared that numerous ambassadors, lamenting with phrases like “Plexus ruined my life,” found themselves struggling financially. This hardship, according to her, was a direct result of the cumulative expenses associated with being a part of Plexus.
Unsubstantiated Claims
Many people are leaving Plexus because their products lack endorsement from reputable scientific and regulatory agencies like the FDA or Health Canada. These endorsements signify that the products have undergone thorough testing and are considered safe and effective. Without such endorsements, there’s no guarantee of safety, and the credibility of the products is in question.
Ethical Concerns
Another reason behind people exiting from Plexus appears to be the discomfort of promoting a product that lacks scientific support or personal belief. This stems from an ethical dilemma, as people generally value honesty and transparency when recommending products to their network. In today’s digital age, one’s reputation is easily affected, and endorsing a product with doubts can harm credibility and relationships, especially if it doesn’t deliver the promised results, as seen with Plexus products.
Pressure
Plexus is losing people because they’re tired of the constant group invites, sales calls, and pressure to recruit. These groups usually just show the good stuff, making it seem like everyone’s doing great, which isn’t always true. Plus, mixing personal stories with sales pitches can feel too pushy for many.
Product Changes and Taste
According to a handful of Reddit threads, Plexus has lost some users because they’ve changed their recipe a few times, changing the taste. Initially, people loved its flavor and health benefits, but recent changes made it taste different and less effective for weight loss. When a familiar product changes too much, especially in taste, it can turn off loyal customers who miss the original experience.
Placebo Effect
A lot of people are ditching Plexus, realizing the benefits they thought they were getting might just be in their heads, not from the product itself. This is all about the placebo effect, where you think something’s working just because you believe it will, even if it’s not actually doing anything.
Lack of FDA Approval
Plexus products aren’t FDA-approved, so it’s hard to be sure if they’re safe or really work. This makes people wonder about what’s in them and if there could be any bad side effects. Basically, without the FDA’s thumbs-up, we don’t have a solid, independent check on Plexus’ claims or their product safety.
Health Risks and Bans
In Australia, they’ve banned some Plexus products because they had DMAA, a banned chemical not listed on the label. Plexus has faced legal issues and safety concerns from regulators. It’s a heads-up that these products might not be great for your health.
Why Is Everyone Leaving Plexus according to Recent Reviews?
People are ditching Plexus for similar reasons folks bail on pyramid schemes. They’re feeling the pinch with high costs, low earnings, and the constant push to get more folks to join. Also, there’s chatter about the pink drink Plexus reviews not being all that great. Plus, some aren’t happy with the products themselves. But hey, Plexus isn’t officially a pyramid scheme; it’s a multi-level marketing (MLM) gig. They make money by selling stuff and adding new sellers.
How do Plexus Pink Drink reviews relate to people leaving Plexus?
Plexus Pink Drink reviews often mention side effects, like in a 2020 ‘Cureus’ study linking it to immune thrombocytopenic purpura (ITP). This negative medical evidence, along with user reviews citing health concerns, is a key reason for people leaving Plexus. In particular, Plexus Pink Drink reviews and the study highlight compounds like garcinia cambogia and chromium polynicotinate as potential causes for these adverse effects, causing concern among users.
Why is everyone leaving Plexus according to Reddit?
Plexus got banned in some countries because some of its products had a thing called dimethylamylamine (DMAA) in them, according to Reddit. DMAA used to be okay for stuff like clearing up your nose, but not anymore. After some scary stories about people getting sick from 1,3-DMAA, lots of places cracked down on it. Canada started the trend in 2011, and then from 2012 to 2013, New Zealand, Australia, Brazil, Finland, Hong Kong, Ireland, Sweden, and the United Kingdom all said no to DMAA or made it super hard to get. So, if you’re on the hunt for Plexus alternatives, you might want to keep that in mind.

